Rule bt0348
[Pathway Prediction Engine]
[All Rules List]
[BBD Main Menu]
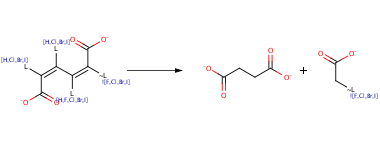
[mono-, di-, or tri-halo]cis,cis-Muconate derivative -> Succinate
tetrahalo-cis,cis-Muconate -> Malonate
[2-halo]Maleylacetate derivative -> Succinate
3,5-Dichloro-2-methylmuconate -> 2-Methyl-3-oxoadipate
| Aerobic Likelihood: | | Likely |
EAWAG-BBD Reaction(s):
Not found
Comments:
This rule handles the cis,cis-muconate (ortho) pathway for the unsubstituted compound, those with a 3-sulfo or 3-carboxy, and most halo derivatives. Most information is found for chloro derivatives; Br and I derivatives are predicted to be degraded in the same way as the Cl derivatives. It includes cycloisomerization of the muconate derivative to form a enelactone (bt0098) or a dienelactone (bt0181 or bt0206), possible isomerization of the enelactone, hydrolysis to form maleylacetate (bt0313), reduction of the double bond (bt0149), addition of CoA, cleavage of acetyl-CoA or haloacetyl-CoA (bt0205), and removal of CoA to form the succinate product. The halide in haloacetate is hydrolytically cleaved to form glycolate. The 2,3,5-trihalodienelactone is predicted to form a halosuccinate, the halide of which is predicted to be hydrolytically cleaved to leave malate, by analogy to chloroacetate. The 2,3,5-trihalomuconate forms the 2,5-dihalodienelactone; further metabolism of this lactone is predicted based on the known metabolism of the 2,3,4,5-tetrachloromuconate. The removal of halogen, carboxyl, and sulfur side groups occurs at different stages of the pathway depending on the substrate (see graphic). This rule also includes the maleylacetate and 2-halomaleylacetate intermediates in this pathway.
Contact Us if you have any comments on rule bt0348.
[Pathway Prediction Engine]
[All Rules List]
[BBD Main Menu]
Page Author(s):
Michael Turnbull
November 20, 2008
This is the EAWAG-BBD biotransformation rule, ruleID# bt0348.
It was generated on July 18, 2025 5:38:25 PM CDT.
© 2025, EAWAG.